Alkyl substituent effects in photochemical and thermal reactions of photochromic thiophene-S,S-dioxidized diarylethenes†
Abstract
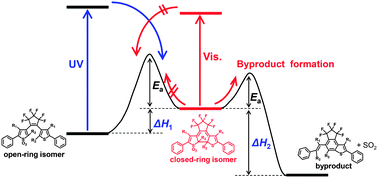
Thiophene-S,S-dioxidized diarylethenes introducing various alkyl groups at the reactive positions were newly synthesized. The diarylethenes showed reversible photochromism, whereas the photocycloreversion reaction was suppressed by thiophene-oxidation. The diarylethene closed-ring isomers with secondary alkyl groups at the reactive positions were found to undergo thermal bleaching reactions to produce byproducts. The thermal bleaching reactions were accelerated by introducing a more bulky substituent at the reactive positions. The relationship between the rate constant of the thermal bleaching reactions and the bulkiness of the substituent at the reactive positions can be correlated using a steric substituent constant. Moreover, it was noted that introduction of a methyl group at the 4-position of the thiophene in the oxidized diarylethenes accelerates the thermal byproduct formation. The rate of the thermal bleaching reactions was found to depend on the difference in the ground state energy between the closed-ring isomer and one of the byproducts, as can be estimated by theoretical calculation of the energy level. Such materials can be used in application such as light-starting irreversible thermosensors.

 Please wait while we load your content...
Please wait while we load your content...