AMVN-initiated expedient synthesis of biaryls by the coupling reaction of unactivated arenes and heteroarenes with aryl iodides†
Abstract
The role of radical initiators AMVN and AIBN has been studied in the potassium tert-butoxide mediated biaryl coupling reaction of aryl iodides with unactivated arenes. Radical initiator AMVN promoted carbon–carbon bond formation expeditiously from aryl iodide having various groups such as amino, methoxy, fluoro, methyl, and trifluoromethyl and arenes in the presence of potassium tert-butoxide (4 equiv.) at 110 °C in 2–5 h. Substituted arenes such as toluene, xylene, anisole, and fluorobenzene also proceeded to form biaryls under AMVN-initiated reaction conditions. Moreover naphthalene, pyridine, pyrimidine, and pyridazine also coupled with aryl iodides and produced biaryls in 41–82% yields. It seems that AMVN initiates the formation of the aryl radical, which enters the radical chain reaction. The generated aryl radical may combine with the arene leading to a biaryl radical, which upon protonation gives the biphenyl radical anion and tert-butanol. The biphenyl radical anion finally reacts with the aryl iodide generating the aryl radical and thus completes the radical chain reaction with concomitant release of biphenyl.
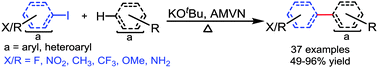

 Please wait while we load your content...
Please wait while we load your content...