Solid-phase synthesis and catalytic sweetening performance of sulfonated cobalt phthalocyanine from sulfonated phthalic anhydride mixture
Abstract
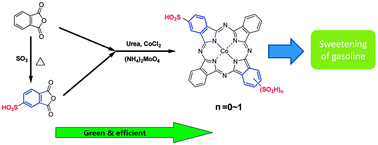
A relatively green, efficient and economical synthetic route for the one-step solid-phase synthesis of sulfonated cobalt phthalocyanine from sulfonated phthalic anhydride mixture was studied, and could solve the serious problems of waste acid pollution and the low utilization of materials in the direct sulfonation of phthalocyanine. The optimal reaction conditions were using a phthalic anhydride mixture with a sulfonation degree of 60% as the raw material, a ratio of urea to PA–SPA mixture of 4 : 1, a ratio of cobalt chloride to PA–SPA mixture of 1 : 4, ammonium molybdate (1 wt%) as catalyst, and carrying out the reaction at 260 °C for 3 hours. Under these optimal conditions, a yield of 52.9% can be achieved. Through sweetening experimental studies, the synthesized sulfonated cobalt(II) phthalocyanine had better sweetening performance than the industrial catalysts, in both the liquid–liquid sweetening and fixed bed sweetening experiments. A sweetening rate of 93% can be achieved in 20 min with the synthesized catalyst in a fixed bed.

 Please wait while we load your content...
Please wait while we load your content...