Synthesis, CMC determination, nucleic acid binding and cytotoxicity of a surfactant–cobalt(iii) complex: effect of ionic liquid additive†
Abstract
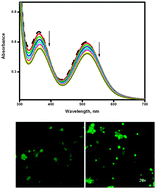
The surfactant–cobalt(III) complex, cis-[Co(trien)(4CNP)(DA)](ClO4)3 (trien = triethylenetetramine, 4CNP = 4-cyanopyridine and DA = dodecylamine) was synthesized and characterized by various spectroscopic and physicochemical techniques. The critical micelle concentration (CMC) value of this surfactant–cobalt(III) complex in aqueous solution was obtained from conductance measurements. The conductivity data (at 303, 308, 313, 318 and 323 K) were used for the evaluation of the temperature-dependent CMC and the thermodynamics of micellization (ΔG0m, ΔH0m and ΔS0m). Absorption, fluorescence, cyclic voltammetry, circular dichroism and viscosity experiments have been carried out to study the interaction of the surfactant–cobalt(III) complex with DNA and RNA. The results suggest that the complex can bind to nucleic acids by intercalation via the long aliphatic chain of the complex into the base pairs of DNA/RNA. In the presence of an ionic liquid additive, the binding strength of the surfactant–cobalt(III) complex to the nucleic acids increased. The complex was tested in vitro on HepG2 (human hepatocellular liver carcinoma) tumor cell lines and found to be active.

 Please wait while we load your content...
Please wait while we load your content...