Addition and in situ halo-cyclization of ω-alkenyl Grignard reagents with aldehydes, ketones, carbon dioxide, and azodicarboxylate†
Abstract
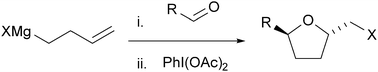
An addition reaction of ω-alkenylmagnesium bromide with aldehydes and consecutive oxidative cyclization with iodobenzene diacetate afforded brominated tetrahydrofuran in one pot. The reaction was also applicable to a one-pot synthesis of 2,5,5-trisubstituted tetrahydrofuran, lactone, and pyrazolidine using a ketone, carbon dioxide, and azodicarboxylate, respectively, as electrophiles. One-pot iodo- and chloro-cyclizations were also possible with alkenylmagnesium iodide and chloride.

 Please wait while we load your content...
Please wait while we load your content...