An extensive investigation of reactions involved in the nitrogen trifluoride dissociation†
Abstract
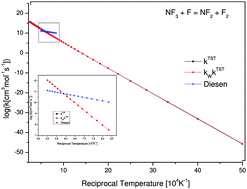
In the course of our studies on nitrogen trifluoride dissociation, we consider in this paper two different sets of reactive systems: the first one consists of the abstraction and the exchange channels of NF3 + F and the other is composed of the unimolecular and the abstraction production of N2 + F. Accurate electronic properties are determined and applied in the scope of the transition state theory (TST) to obtain thermal rate constants for these systems. An extensive investigation in terms of minimum energy paths and intrinsic reaction coordinates was previously carried out in order to ensure the good quality of our TST results. We apply Wigner corrections to consider tunneling effects whenever their importance is numerically verified. The obtained results for the abstraction channel thermal rate constants are in good agreement with

 Please wait while we load your content...
Please wait while we load your content...