O2activation and external substrate oxidation capability of a Co(ii)–semiquinonato complex†
Abstract
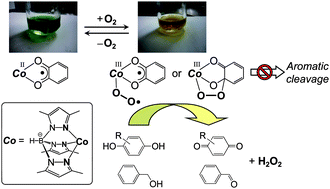
The synthesis, characterization and catalytic oxidizing activity of a cobalt(II)–semiquinonato complex with hydrotris(3,5-dimethyl-1-pyrazolyl)borate (=TpMe2) have been investigated. The cobalt(II)–semiquinonato complex can be synthesized by two alternative routes; (i) dehydrative condensation of a cobalt(II)–hydroxo complex with

 Please wait while we load your content...
Please wait while we load your content...