Reusable α-MoO3 nanobelts catalyzes the green and heterogeneous condensation of 1,2-diamines with carbonyl compounds†
Abstract
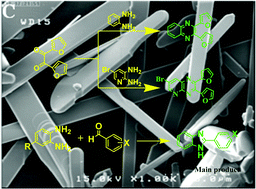
Crystalline nanobelts of α-MoO3 have been obtained successfully using novel and safe agents through a simple and safe sol–gel method (polymerizing–complexing) and characterized by

 Please wait while we load your content...
Please wait while we load your content...