Regioselective N-alkylation with alcohols for the preparation of 2-(N-alkylamino)quinazolines and 2-(N-alkylamino)pyrimidines†
Abstract
In the presence of the [Cp*IrCl2]2/NaOH system, the direct N-alkylation of 2-aminoquinazolines and 2-aminopyrimidines with
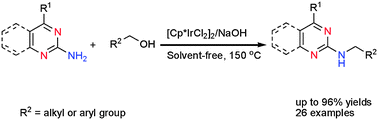

 Please wait while we load your content...
Please wait while we load your content...