Direct anti and regio-specific aldol reactions of cyclododecanone catalyzed by alkali metal hydroxides: implications for supramolecular helical design†
Abstract
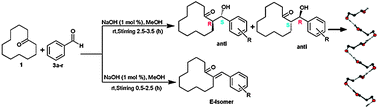
The direct ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, electrostatic interaction between aldehyde substituents and metal enolates and the bulkiness of the benzaldehyde ring substituents. We extensively optimized the reaction conditions using different
O groups, electrostatic interaction between aldehyde substituents and metal enolates and the bulkiness of the benzaldehyde ring substituents. We extensively optimized the reaction conditions using different

 Please wait while we load your content...
Please wait while we load your content...