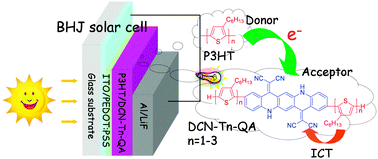
A series of regioregular head-to-tail oligo(3-hexylthiophene)-functionalized dicyano-ethylene substituted quinacridone derivatives DCN-Tn-QA (n = 1–3) were designed and synthesized. These linear π-conjugated donor–acceptor–donor (D–A–D) molecules with different length of the oligothiophene chain have been achieved in good yields via iterative iodination and Suzuki cross-coupling reactions followed by Knoevenagel condensations. Their photophysical and electrochemical properties, as well as density functional theory (DFT) calculations, were systematically investigated. The compounds of DCN-Tn-QA (n = 1–3) with an intense absorption range from 350–750 nm, suitable LUMO levels at around −4.0 eV and considerable film formation properties, were suitable for application in organic solar cells (OSCs) as acceptors. The organic bulk heterojunction (BHJ) solar cell based on the blend film of P3HT/DCN-T2-QA (1 : 1, w/w) (P3HT = poly(3-hexylthiophene)) showed a power conversion efficiency (PCE) of 0.51% under the illumination of AM.1.5, 100 mW cm−2. Compared with P3HT/PCBM (PCBM = [6,6]-phenyl-C61-butyric acid methyl ester), the blend film of P3HT/DCN-T2-QA displayed a stronger response to the solar spectrum in the long wavelength range from 665–750 nm.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...