π-Complexed polyfluoroarenes: a reactivity, bonding and spectroscopic study of (η6-C6F6)Cr(η6-C6H6) and related molecules†‡
Abstract
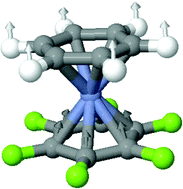
The syntheses, structures and reactivity of fluorinated di-arene chromium sandwich complexes (chromarenes) are reviewed, and their bonding characteristics probed by an analysis of their spectroscopic properties. In particular, density functional theory, together with a detailed vibrational study of (C6F6)Cr(C6H6), has been used to study a system containing the very rare hexahapto-coordinated hexafluorobenzene
- This article is part of the themed collection: In honour of Didier Astruc

 Please wait while we load your content...
Please wait while we load your content...