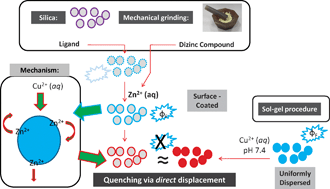
Herein, we have explored expeditious and lowered expense SiO2-loading in the context of fluorescent “off–on” and “on–off” Zn2+ ion detection/controlled release. A simple racemic ligand (H2rlys) (1) prepared in situ can be mechanically SiO2-loaded so as to induce surface sites that allow for “off–on” aqueous fluorescence (λem,max = ∼450 nm) detection of Zn2+; with Cu2+ as a competing input, “inhibit” logic gate behaviour is present. Oppositely, dimeric, chiral fluorescent zinc complexes (ΦF = ∼0.20) prepared in a “one pot” method, and bearing different Zn⋯Zn internuclear spacings, can be incorporated into silica by two methods. Specifically, the chiral Zn2(slysH)2Cl2 (2) and racemic Zn2(rslysH)2(μ-OAc)2 species (3) [slys or rslys = 6-amino-2-{(2-hydroxybenzylidene)amino}hexanoate] were mechanochemically loaded. An adsorption constant was 1.99 × 10−4 (molecules of 2/units SiO2)(min)−1 for times of 5–20 min, as determined by extensive SEM-EDS data. These confirmed the highly selective “on–off” rapid aqueous fluorescent Cu2+ detection (versus, Li+, Na+, Cs+, Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Ni2+, Ag+, Cd2+, Hg2+, Pb2+) through direct Zn2+ displacement under bulk neat water flow (pH = 7.4) as verified by an authentic sample of [Cu2(slysH)2(NO3)2]–silica (4–silica). With the use of 2·SiO2, Na4EDTA and Cu2+ “inhibit” logic gate behaviour was also found. Sol–gel techniques allowed for loadings of 2 (20–890 mg) up to 1 ∶ 1 (by wt). The preparation of these inorganic hybrids are extremely inexpensive and rapid to prepare from commercial starting materials and can be extended to variegated forms of SiO2; alumina gel was also functionalized with 2. Cu2+ and Zn2+ are metals pertinent to human neurological health promotion and in neurodegenerative diseases.

 Please wait while we load your content...
Please wait while we load your content...