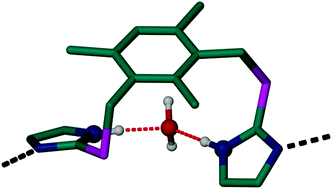
A novel conformationally flexible ditopic ligand 1,3-bis(1-imidazolyl-2-thione)-2,4,6-trimethylbenzene (L), bearing thioether linkages between a central aromatic moiety and two pendant imidazole rings, was synthesised. Treatment of L with CdI2 under varying conditions afforded four new solvates, {[CdLI2]·H2O}n, {[CdLI2]·CH3OH}n, {[CdLI2]·2CH3CN}n, and {[CdLI2]·2CH3OH}n, which were characterised by single crystal X-ray diffraction. In all four of the complexes the Cd metal centers are tetrahedrally coordinated to two iodide ions and two nitrogen atoms from separate L molecules to form continuous 1D polymeric strands. The solvent molecules participate in strong hydrogen bonding with the amino nitrogen atoms of the imidazole rings. Hirshfeld surface analysis and breakdown of the corresponding 2D fingerprint plots of the four structures provide a convenient means of quantifying the interactions within the crystal structures, revealing significant similarities in the interactions experienced by each complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...