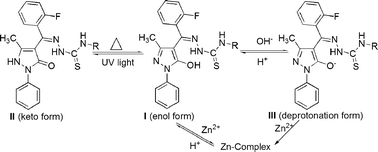
Two novel compounds containing a pyrazolone-ring unit, i.e.1-phenyl-3-methyl-4-(2-fluorobenzal)-5-hydroxypyrazole 4-methylthiosemicarbazone and 1-phenyl-3-methyl-4-(2-fluorobenzal)-5-hydroxypyrazole 4-ethylthiosemicarbazone, have been synthesized and characterized by MS, IR, 1H NMR spectra and X-ray single crystal diffraction. In solid state, they exhibit reversible photochromic properties under UV light irradiation and heating. Based on the crystal structure, solid IR spectra and theoretical calculation, the photochromic mechanism of the intra- and intermolecular double-proton transfer from the enol form to the keto form is proposed. In solution, stimulated by three chemical inputs (H+, OH− and Zn2+), they undergo the deprotonation–protonation and complexation reactions. Based on an absorption band at 355 nm as the output signal, an INHIBIT logic gate combining a NOT and an AND gate has been obtained.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...