What can quantum chemistry tell us about Pa(v) hydration and hydrolysis?†
Abstract
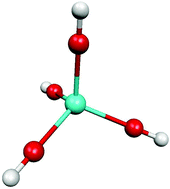
Published liquid–liquid extraction studies of Pa(V) were interpreted with aqueous mono-, di- and trications. B3LYP DFT is applied here to such cations surrounded by two explicit hydration layers: Linear or tetrahedral geometries are found for the Pa(V) aquo ions. PaO2+ is similar to the other AnO2+ cations, but has strong apical bonds, resulting from the highly negative Oyl charge, which decreases along the An(V) series. This explains the instability of PaO2+ in water, and the differences with the heavier An(V). PaO2+ diprotonates to give Pa(OH)23+ and can further dihydrolyse to give Td-Pa(OH)4+, which might very well be the most stable Pa(V) monocation. PaOOH2+ is confirmed to be the Pa(V) aqueous dication invoked in the literature for pH ≤ 1.4 ± 0.7. PaO3+ is confirmed in sulfate solution, with a bond length close to 180 pm. Pa(OH)23+ cannot be excluded in other conditions. The strong influence of the solvent was not fully taken into account in most previous theoretical studies that focused only on bare or partially solvated PaO2+. Toraishi et al. have studied hydrated Pa(V) and our work confirms this study and its qualitative interpretation. The new tetrahedral Pa(OH)4+ geometry that is shown here to be important opens the field to further quantum chemical studies of Pa(V) and other f-elements. As a test for the two-shell model approach for Pa(V), fluoride coordination to Pa(V) is studied and compared with published EXAFS data: an excellent fit is obtained with the well-established species PaF72−, but most other stoichiometries tested are precluded.

 Please wait while we load your content...
Please wait while we load your content...