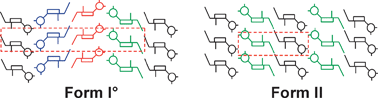
This work highlights the structural and thermochemical differences of two modifications of the NK1receptor antagonist aprepitant. Form I° is the stable polymorph and crystallises in the orthorhombic space groupP212121 whereas the metastable form II is monoclinic (space groupP21). The monotropically related polymorphs show only minor differences in melting point and heat of fusion (Tfus,I = 253.6, ΔfusHI = 53.7 kJ mol−1, Tfus,II = 253.0 °C, ΔfusHII = 52.4 kJ mol−1) and often crystallise concomitantly. The forms exhibit a very close structural relationship based on a common 2D packing fragment, which is in fact a stack of 1D N–H⋯O hydrogen bonded ribbon chains. Forms I° and II may therefore be interpreted as two distinct stacking modes of this common 2D unit. The alternative modes are associated with slight differences in weaker intermolecular interactions. Somewhat surprisingly, the aprepitant molecule adopts almost the same conformation in the two crystal structures in spite of its potential conformational flexibility. Hirshfeld surface analysis was successfully deployed to visualise and elaborate the small differences in the molecular environments of the two polymorphs. The study emphasises the benefit of single-crystal structure data for the judgement of the phase purity and of polymorphs exhibiting only weak energetical and structural differences.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...