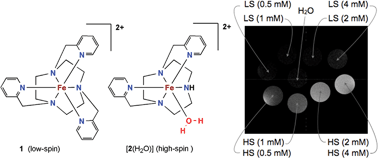
We have identified a pair of structurally similar iron complexes in the oxidation state II that exist in a low-spin and a high-spin electronic spin state in aqueous media, respectively. The low-spin, diamagnetic complex (LS, 1) is mute in MRI while the high-spin, paramagnetic complex (HS, 2) generates considerable contrast in MRI. These results demonstrate that iron(II) complexes, hitherto neglected for contrast enhancement in MRI, have potential for the design of an MRI probe that suffers passage from one state to the other under the influence of a targeted biochemical activity and thus operates in an off–on mode. At 300 MHz (proton resonance frequency at 7 T field strength) and in phosphate buffer, we found a longitudinal relaxivity (r1) of 1.29 mM−1 s−1 for 2 that, in light of the difference in unpaired electrons of the central metal atoms (4 for FeII; 7 for GdIII), comes remarkably close to that of gadolinium(III)–DOTA (2.44 mM−1 s−1), a commercialized MRI contrast agent. Since gadolinium complexes are always paramagnetic and can therefore not be muted in MRI, the here presented Fe(II)-based system offers an alternative strategy to develop responsive MRI probes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...