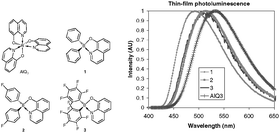
A series of boron quinolinate compounds with different degrees of fluorination {Ph2BQ 1, (4-F-C6H4)2BQ 2 and (C6F5)2BQ 3 (where Q is 8-quinolinate)} have been prepared and their electronic and luminescent behaviour has been investigated in a variety of organic light emitting device structures. Cyclic voltammetry studies have shown a decrease in ionization potential with the degree of fluorination. Electroluminescence (EL) measurements have shown increasingly red-shifted exciplex emission, originating from the different boron compounds interacting with the hole transporting layer. In layered devices, the boron compounds 1–3 are inferior in their EL performance compared to aluminium tris(8-quinolinoate) (AlQ3). However, when the boron compounds 1, 2 or 3 are doped into a 4,4′-bis(carbazol-9-yl)diphenyl (CBP) host, emission solely attributable to 1–3 is observed. In such devices, the boron compounds 1 and 2 outperform AlQ3 as an emitter at low to moderate current densities.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...