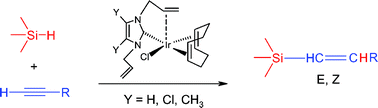
A family of alkenyl-functionalized N-heterocyclic-carbene–iridium(I) complexes has been synthesized, providing a series of mono-coordinated, bis-chelate and pinceralkenyl-NHC species. Olefin coordination is highly influenced by the nature of the substituents on the NHC ring, and on the length of the alkenyl branch. A fluxional process involving coordination/decoordination of the olefin in bis-allyl-NHC complexes has been studied, and the activation parameters have been determined by means of VT-NMR spectroscopy. The mono-coordinated complexes are highly active in the hydrosilylation of terminal alkynes, showing high selectivity for the Z-isomers, with no α-isomers or dehydrogenative silylation processes being observed. The molecular structures reported that are representative of the species have been determined by means of X-ray crystallography.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...