Au(iii)-catalyzed ring opening reaction of 1-cyclopropyl-2-yn-1-ols with nucleophiles: highly efficient approach to (Z)-conjugated enynes†
Abstract
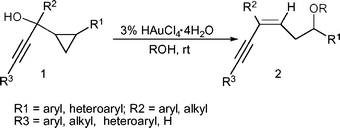
A highly efficient approach to (Z)-conjugated enynes has been developed by utilizing an Au(III)-catalyzed ring opening reaction of 1-cyclopropyl-2-yn-1-ols with
 Please wait while we load your content...
Please wait while we load your content...