Dansylated resorcinarenes
Abstract
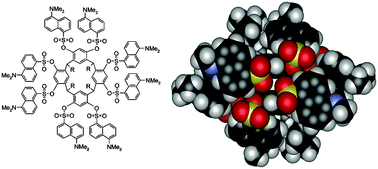
The synthesis, X-ray crystal structures and spectroscopic characterization (UV-Vis absorption and fluorescence emission) of regioselective tetradansylated resorcinarene 2 and octadansylated resorcinarene 3 are described. In solution, the resorcinarene backbone of 2 shows a boat conformation with C2v symmetry, while the octasubstituted 3 shows at room temperature a time-averaged C4v symmetry, which turns into stable boat conformation at low temperatures. The four hydroxyl groups of 2, not present in 3, form hydrogen bonds to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonds. In addition 2 forms a directly hydrogen-bonded dimeric assembly via four intermolecular O–H⋯O and O–H⋯O
S hydrogen bonds. In addition 2 forms a directly hydrogen-bonded dimeric assembly via four intermolecular O–H⋯O and O–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S hydrogen bonds. In the solid state both 2 and 3 exhibit a multitude of intra- and intermolecular π–π interactions between the adjacent dansyl moieties and also with the aromatic parts of the macrocyclic skeleton. The absorption and fluorescence spectra of 2 and 3, together with a reference compound, dansylphenolate 4, were recorded. In chloroform, with the same dansyl concentration and absorption at excitation wavelength, fluorescence from 4 was clearly stronger than that from either 2 or 3, an indication of excited-state dansyl–dansyl interactions in the dansyl substituted resorcinarenes. The absorption and emission bands of the dansyl units were gradually substituted by the absorption and emission bands of the protonated dansyl units when 2 and 3 were titrated with
S hydrogen bonds. In the solid state both 2 and 3 exhibit a multitude of intra- and intermolecular π–π interactions between the adjacent dansyl moieties and also with the aromatic parts of the macrocyclic skeleton. The absorption and fluorescence spectra of 2 and 3, together with a reference compound, dansylphenolate 4, were recorded. In chloroform, with the same dansyl concentration and absorption at excitation wavelength, fluorescence from 4 was clearly stronger than that from either 2 or 3, an indication of excited-state dansyl–dansyl interactions in the dansyl substituted resorcinarenes. The absorption and emission bands of the dansyl units were gradually substituted by the absorption and emission bands of the protonated dansyl units when 2 and 3 were titrated with

 Please wait while we load your content...
Please wait while we load your content...