Long-lived and highly conducting ion channels formed by lipophilic ethylenediamine palladium(ii) complexes†
Abstract
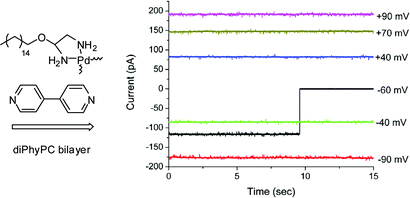
The synthesis of a lipophilic ethylenediamine palladium(II) complex and its activity in planar bilayer membranes are reported. In acetonitrile solution containing one equivalent of
- This article is part of the themed collection: In honour of George Gokel

 Please wait while we load your content...
Please wait while we load your content...