Reaction of NbCl3(MeOCH2CH2OMe)(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe) with KTp4Bo,3Me in THF produces a 68% yield of the dichloro–phenylpropyne complex Tp4Bo,3MeNbCl2(PhC
CMe) with KTp4Bo,3Me in THF produces a 68% yield of the dichloro–phenylpropyne complex Tp4Bo,3MeNbCl2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe) [Tp4Bo,3Me = hydrotris(3-methylindazol-1-yl)borate]. As observed by solution NMR spectroscopy, the four-electron donor alkyne sits in the molecular mirror plane and restricted rotation of the alkyne ligand allows the observation of an equilibrium between two rotamers. The conformation of the alkyne ligand in the major isomer is such that the phenyl group is proximal to Tp4Bo,3Me. Unexpectedly, the minor rotamer in solution, that with the distal phenyl group, is observed in the crystal. Analysis of the possible interactions suggest that aromatic interactions are responsible for this unusual observation.
CMe) [Tp4Bo,3Me = hydrotris(3-methylindazol-1-yl)borate]. As observed by solution NMR spectroscopy, the four-electron donor alkyne sits in the molecular mirror plane and restricted rotation of the alkyne ligand allows the observation of an equilibrium between two rotamers. The conformation of the alkyne ligand in the major isomer is such that the phenyl group is proximal to Tp4Bo,3Me. Unexpectedly, the minor rotamer in solution, that with the distal phenyl group, is observed in the crystal. Analysis of the possible interactions suggest that aromatic interactions are responsible for this unusual observation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe) with KTp4Bo,3Me in THF produces a 68% yield of the dichloro–phenylpropyne complex Tp4Bo,3MeNbCl2(PhC
CMe) with KTp4Bo,3Me in THF produces a 68% yield of the dichloro–phenylpropyne complex Tp4Bo,3MeNbCl2(PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe) [Tp4Bo,3Me = hydrotris(3-methylindazol-1-yl)borate]. As observed by
CMe) [Tp4Bo,3Me = hydrotris(3-methylindazol-1-yl)borate]. As observed by 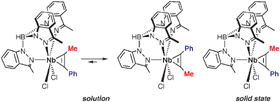

 Please wait while we load your content...
Please wait while we load your content...