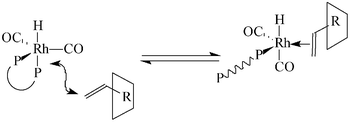
Interaction between α-cyclodextrin or β-cyclodextrin and the alkyl sulfonated diphosphines DPPETS (tetrasulfonated 1,2-bis(diphenylphosphino)ethane), DPPPTS (tetrasulfonated 1,3-bis(diphenylphosphino)propane) and DPPBTS (tetrasulfonated 1,4-bis(diphenylphosphino)butane) was investigated by NMR and UV-Visible spectroscopy. Contrary to α-cyclodextrin, β-cyclodextrin forms inclusion complexes with each sulfonated diphosphine. Continuous variation plots and titration experiments obtained from 1H and 31P NMR data indicated the formation of 1∶1 inclusion complexes and allowed the calculation of the association constants for each complex. Information on the structures of these complexes were obtained by T-ROESY NMR experiments. The potential of these ligands associated with methylated α- or β-cyclodextrin during the reaction of hydroformylation of 1-decene was studied. In all cases, the presence of cyclodextrins increased the conversion and the chemoselectivity whereas the linear to branched ratio of the aldehyde product decreased. This decrease in regioselectivity was attributed to the formation of low-coordinated phosphine species. In fact, in order to reduce steric hindrance around the metal center, a mono-dissociation of the bidentate ligand seemed to occur when the inclusion complex between the cyclodextrin and 1-decene approached the metal center. In the case of methylated-β-cyclodextrin, the phenomenon is accentuated since one of the two diphenylphosphino groups of the bidentate could be trapped by this cyclodextrin leading to the formation of a second-sphere coordination adduct.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...