H2O2 evolution during the photocatalytic degradation of organic molecules on fluorinated TiO2
Abstract
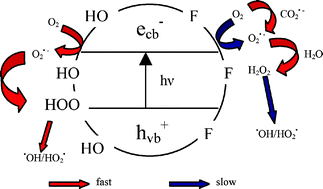
The effect of TiO2 surface fluorination on the hydrogen peroxide evolution occurring in photocatalytic runs was investigated employing the azo dye Acid Red 1 (AR1) and two model organic molecules with acidic properties, i.e. formic acid (FA) and benzoic acid (BA), as substrates of oxidative degradation. While AR1 and BA photocatalytic degradation on fluorinated titanium dioxide (F–TiO2) was markedly faster than on unmodified TiO2, because of enhanced hydroxyl radical formation, H2O2 concentration during the photodegradation of both substrates on F–TiO2 was lower, possibly because of the reduced rate of interfacial electron transfer. By contrast, FA underwent slower photocatalytic degradation on F–TiO2, but, at the same time, hydrogen peroxide concentration was relatively high, while no H2O2 could be detected during FA photodegradation on unmodified TiO2. Photocatalytic runs in the presence of the nitrate anion, able to react with the CO2˙− species produced from FA oxidation, but not with conduction band electrons, demonstrated that CO2˙− plays a relevant role in H2O2 formation during FA degradation on F–TiO2. In fact, surface fluoride, having a shielding effect at the semiconductor–water interface, not only inhibits the photocatalytic decomposition of H2O2, but also favours CO2˙− desorption and reaction with dissolved O2, generating H2O2. By contrast, CO2˙− mainly gives electron transfer to the conduction band of naked TiO2 and surface reduction of the photocatalytically produced H2O2.

 Please wait while we load your content...
Please wait while we load your content...