Catalytic synthesis of methanethiol from CO/H2/H2S mixtures using α-Al2O3
Abstract
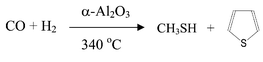
Sustained synthesis of methanethiol from the reaction of CO/H2/H2S mixtures is reported and discussed. Surprisingly, unmodified α-Al2O3 gives the best results for this reaction and methanethiol selectivities of >98% at CO conversions of ca. 6% can be readily obtained (CO∶H2∶H2S = 4∶5∶1, 340 °C, total pressure = 20 bar, 200 h−1). Reaction of CO + H2 (CO∶H2 = 1∶1) in the absence of H2S using α-Al2O3 under comparable conditions gives a lower CO conversion (ca. 1.3%) with significant selectivities to methane (20%), methanol (28.5%) and ethanol (21.1%). When H2S is added to the synthesis gas feedstock, the product selectivity switches to sulfur-containing products, almost exclusively methanethiol, but some by-product thiophene is also observed. A range of other catalysts were also investigated (e.g., γ-Al2O3, Cr2O3, Cr2O3/Al2O3, Cu/Cr2O3) but all give inferior catalytic performance when compared with α-Al2O3. The mechanism of the synthesis of methanethiol is discussed, based on a modification of chain propagation in the Fischer–Tropsch synthesis reaction.

 Please wait while we load your content...
Please wait while we load your content...