Basicity of lactones and cyclic ketones towards I2 and ICl. An experimental and theoretical study†
Abstract
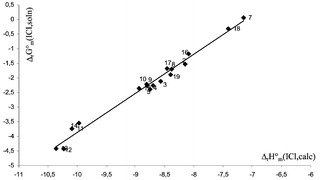
Intermolecular charge transfer (CT) spectra of several complexes between cyclic ketones and lactones and molecular iodine and iodine monochloride were studied in the UV-visible region. Equilibrium constant and free energy changes of the formed complexes were determined in solution. Ab initio calculations at HF/LANL2DZ* and MP2(full)/LANL2DZ* were carried out to establish the nature of the complexation site in the case of lactones, to determine the complex structures and to examine the ring size effect. Although range of the basicity towards I2 and ICl of compounds studied was small, it was found that cyclic ketones are more basic than lactones. This basicity difference decreases from small to large cycles and practically vanishes for six- and seven-membered rings. A comparative analysis between basicities of lactones and aliphatic esters towards I2 and ICl has also been carried out. Experimental data in solution were found to be linearly correlated with theoretical results in the gas phase. Proton affinities of cyclic ketones and aliphatic carbonyl compounds that do not present any secondary interaction with the Lewis acid correlate very well with their gas-phase basicity towards I2 and ICl.

 Please wait while we load your content...
Please wait while we load your content...