Nitro derivatives of pyrrole, furan and 1H-tetrazole: ring or nitro bases?
Abstract
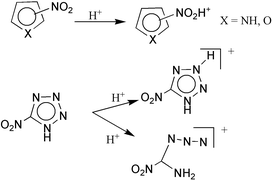
The gas-phase basicity of pyrrole, furan, 1H-tetrazole and their nitro derivatives has been analyzed by means of high level ab initio and DFT calculations. The gas-phase basicity of 2-nitrofuran was also determined by means of FT-ICR mass spectrometry. Our results indicate that although pyrrole and furan behave as carbon bases in the gas phase, their nitro derivatives protonate preferentially on the nitro group. Conversely, both 1H-tetrazole and its 5-nitro derivative protonate preferentially on the ring, because the intrinsic basicity of the nitro group is significantly dampened when the group is attached to a tetrazolic ring. For the 5-nitro-1H-tetrazole the most stable protonated species corresponds to an open structure formed by protonation on N1, which is followed by a N1–N2 bond cleavage. This non-cyclic structure is entropically favored and therefore it should be the one observed in ICR measurements.

 Please wait while we load your content...
Please wait while we load your content...