Chemical induced fragmentation of MOFs for highly efficient Ni-based hydrogen evolution catalysts†
Abstract
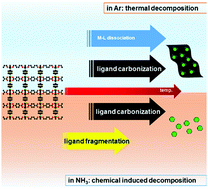
A highly efficient catalyst for the hydrogen evolution reaction (HER) composed of Ni nanoparticles encapsulated in thin layers of N-doped carbon (Ni@NC) can be obtained by NH3 induced fragmentation of a Ni based MOF at mild temperature (450 °C). A mechanistic study shows that the high chemical reactivity of NH3 causes ammonolysis of the ligands and allows ligand removal well below their carbonization temperature. In conventional thermal decomposition in Ar, metal–ligand bond dissociation and ligand carbonization occurs in the same temperature region, resulting in a catalyst with high carbon content and much poorer HER performance. We further improve the HER performance by combining the NH3 induced fragmentation and destabilization of the Ni-MOF structure by low levels of Co substitution, which results in smaller, more uniform catalyst particles. Using the noble metal free HER catalyst, an overall solar-to-hydrogen efficiency of 7.9% is achieved in an electrolyzer driven by a commercial polycrystalline solar cell with an efficiency of 12.9%.


 Please wait while we load your content...
Please wait while we load your content...