Alloy oxidation as a route to chemically active nanocomposites of gold atoms in a reducible oxide matrix†
Abstract
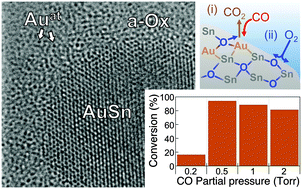
While nanoparticles are being pursued actively for a number of applications, dispersed atomic species have been explored far less in functional materials architectures, primarily because composites comprising dispersed atoms are challenging to synthesize and difficult to stabilize against sintering or coarsening. Here we show that room temperature oxidation of Au–Sn alloys produces nanostructures whose surface is terminated by a reducible amorphous oxide that contains atomically dispersed Au. Analysis of the oxidation process shows that the dispersal of Au in the oxide can be explained by predominant oxygen anion diffusion and kinetically limited metal mass transport, which restrict phase separation due to a preferential oxidation of Sn. Nanostructures prepared by oxidation of nanoscale Au–Sn alloys with intermediate Au content (30–50%) show high activity in a CO-oxidation probe reaction due to a cooperative mechanism involving Au atoms as sites for CO adsorption and reaction to CO2 embedded in a reducible oxide that serves as a renewable oxygen reservoir. Our results demonstrate a reliable approach toward nanocomposites involving oxide-embedded, atomically dispersed noble metal species.

 Please wait while we load your content...
Please wait while we load your content...