The G-M-N motif determines ion selectivity in the yeast magnesium channel Mrs2p
Abstract
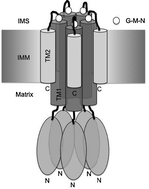
The highly conserved G-M-N motif of the CorA-Mrs2-Alr1 family of Mg2+ channels has been shown to be essential for Mg2+ transport. We performed random mutagenesis of the G-M-N sequence of Saccharomyces cerevisiae Mrs2p in an unbiased genetic screen. A large number of mutants still capable of Mg2+ influx, albeit below the wild-type level, were generated. Growth complementation assays, performed in media supplemented with Ca2+ or Co2+ or Mn2+ or Zn2+ at varying concentrations, lead to identification of mutants with reduced growth in the presence of Mn2+ and Zn2+. We hereby conclude that (1) at least two, but predominantly all three

 Please wait while we load your content...
Please wait while we load your content...