Control of the stereo-selectivity of styreneepoxidation by cytochrome P450 BM3 using structure-based mutagenesis†‡
Abstract
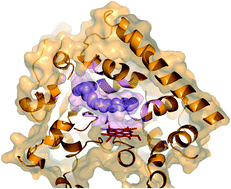
The potential of flavocytochrome P450 BM3 (CYP102A1) from Bacillus megaterium for biocatalysis and biotechnological application is widely acknowledged. The catalytic and structural analysis of the Ala82Phe mutant of P450 BM3 has shown that filling a hydrophobic pocket near the active site improved the binding of small molecules, such as
- This article is part of the themed collection: Cytochromes

 Please wait while we load your content...
Please wait while we load your content...