The role of His-50 of α-synuclein in binding Cu(ii): pH dependence, speciation, thermodynamics and structure†‡
Abstract
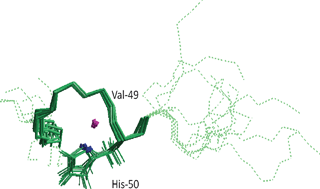
Copper interaction with alpha synuclein (αS) has been shown to accelerate aggregation and oligomerization of the
- This article is part of the themed collection: Metals in Neurodegenerative Disease

 Please wait while we load your content...
Please wait while we load your content...