Systems analysis of the genetic interaction network of yeast molecular chaperones†
Abstract
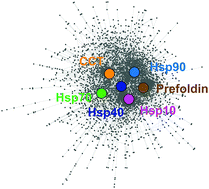
Molecular chaperones are typically promiscuous interacting proteins that function globally in the cell to maintain protein homeostasis. Recently, we had carried out experiments that elucidated a comprehensive interaction network for the core 67 chaperones and 15 cochaperones in the budding yeast Saccharomyces cerevisiae [Rizzolo et al., Cell Rep., 2017, 20, 2735–2748]. Here, the genetic (i.e. epistatic) interaction network obtained for chaperones was further analyzed, revealing that the global topological parameters of the resulting network have a more central role in mediating interactions in comparison to the rest of the proteins in the cell. Most notably, we observed Hsp10, Hsp70 Ssz1 chaperone, and Hsp90 cochaperone Cdc37 to be the main drivers of the network architecture. Systematic analysis on the physicochemical properties for all chaperone interactors further revealed the presence of preferential domains and folds that are highly interactive with chaperones such as the WD40 repeat domain. Further analysis with established cellular complexes revealed the involvement of R2TP chaperone in quaternary structure formation. Our results thus provide a global overview of the chaperone network properties in yeast, expanding our understanding of their functional diversity and their role in protein homeostasis.


 Please wait while we load your content...
Please wait while we load your content...