Synthesis and antifungal activity of novel oxazolidin-2-one-linked 1,2,3-triazole derivatives†
Abstract
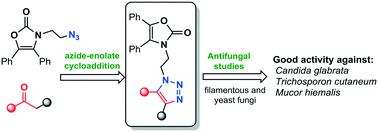
Novel oxazolidin-2-one-linked 1,2,3-triazole derivatives (4a–k) were synthesized by straightforward and versatile azide–enolate (3 + 2) cycloaddition. The series of compounds was screened for antifungal activity against four filamentous fungi as well as six yeast species of Candida spp. According to their efficiency and breadth of scope, they can be ordered as 4k > 4d > 4h > 4a, especially in relation to the activity displayed against Candida glabrata ATCC-34138, Trichosporon cutaneum ATCC-28592 and Mucor hiemalis ATCC-8690, i.e. compounds 4d, 4h and 4k showed excellent activity against C. glabrata (MIC 0.12, 0.25 and 0.12 μg mL−1, respectively), better than that of itraconazole (MIC 1 μg ml−1). The activity of compound 4d (MIC = 2 μg mL−1) was higher than that observed for the standard antifungal drug (MIC = 8 μg mL−1) against Trichosporon cutaneum, while compound 4k displayed an excellent antimycotic activity against Mucor hiemalis (MIC = 2 μg mL−1vs. 4 μg mL−1 for itraconazole). In addition, we describe herein a novel mild and eco-friendly synthetic protocol for obtaining β-ketosulfones (adducts to afford compounds 4a–k) from α-brominated carbonyls in an aqueous nanomicellar medium at room temperature.


 Please wait while we load your content...
Please wait while we load your content...