Pyrimidine nucleotides containing a (S)-methanocarba ring as P2Y6 receptor agonists†
Abstract
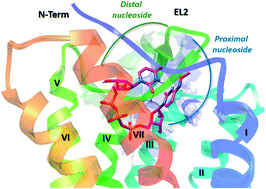
Both agonists and antagonists of the UDP-activated P2Y6 receptor (P2Y6R) have been proposed for therapeutic use, in conditions such as cancer, inflammation, neurodegeneration and diabetes. Uracil nucleotides containing a south-bicyclo[3.1.0]hexane ((S)-methanocarba) ring system in place of the ribose ring were synthesized and shown to be potent P2Y6R agonists in a calcium mobilization assay. The (S)-methanocarba modification was compatible with either a 5-iodo or 4-methoxyimino group on the pyrimidine, but not with a α,β-methylene 5′-diphosphate. (S)-Methanocarba dinucleotide potency was compatible with a N4-methoxy modification on the proximal nucleoside that is assumed to bind at the P2Y6R similarly to UDP; (N)-methanocarba was preferred on the distal nucleoside moiety. This suggests that the distal dinucleotide P2Y6R binding site prefers a ribose-like group that can attain a (N) conformation, rather than (S). Dinucleotide binding was modeled by homology modeling, docking and molecular dynamics simulations, which suggested the same ribose conformational preferences found empirically.


 Please wait while we load your content...
Please wait while we load your content...