PAIN-less identification and evaluation of small molecule inhibitors against protein tyrosine phosphatase 1B†‡
Abstract
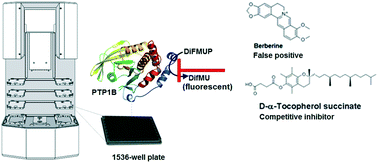
A highly miniaturized biochemical assay was set up to test a focused set of natural products against the enzymatic activity of protein tyrosine phosphatase 1B (PTP1B). The screen resulted in the identification of the natural product alkaloids, berberine and palmatine as well as α-tocopheryl succinate (α-TOS) as potential inhibitors of PTP1B. In a second step, several read-out and counter assays were applied to confirm the observed inhibitory activity of the identified hits and to remove false positives which target the enzymatic activity of PTP1B by a non-specific mechanism, also known as PAINS (pan-assay interference compounds). Both, berberine and palmatine were identified as false positives which interfered with the assay read-out. Using NMR spectroscopy, self-association via stacking interactions was detected for berberine in aqueous media, which may also contribute to the non-specific inhibition of PTP1B. α-TOS was confirmed as a novel reversible and competitive inhibitor of PTP1B. A concise structure–activity relationship study identified the carboxyl group and the saturated phytyl-side chain as being critical for PTP1B inhibition.


 Please wait while we load your content...
Please wait while we load your content...