Synthesis of novel inhibitors of α-amylase based on the thiazolidine-4-one skeleton containing a pyrazole moiety and their configurational studies†
Abstract
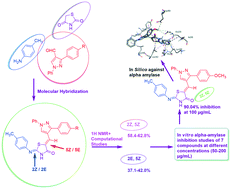
Postprandial hyperglycemia can be controlled by delaying the absorption of glucose resulting from carbohydrate digestion. α-Amylase is the initiator of the hydrolysis of polysaccharides, and therefore developing α-amylase inhibitors can lead to development of new treatments for metabolic disorders like diabetes mellitus. In the present work, we set out to rationally develop α-amylase inhibitors based on the thiazolidine-4-one scaffold. The structures of all these newly synthesized hybrids were confirmed by spectroscopic analysis (IR, 1H-NMR, MS). The appearance of two sets of signals for some protons in 1H NMR revealed the existence of a mixture of 2E,5Z (37.1–42.0%) and 2Z,5Z isomers (58.4–62.8%), which was further supported by DFT studies. All the newly synthesized compounds have potential inhibitory properties as revealed through in vitro α-amylase inhibition activity. Compound 5a at 100 μg mL−1 concentration showed a remarkable inhibition of 90.04%. In vitro α-amylase inhibition was further supported by docking studies of compound 5a against the active site of human pancreatic α-amylase (PDB ID: 2QV4). The docking studies revealed that the bonding interactions found between 5a and human pancreatic α-amylase are similar to those responsible for α-amylase inhibition by acarbose.


 Please wait while we load your content...
Please wait while we load your content...