Design, synthesis and biological evaluation of imidazopyridine–propenone conjugates as potent tubulin inhibitors†‡
Abstract
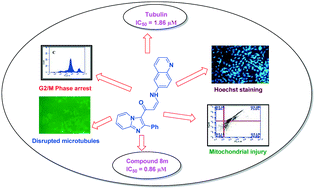
A library of imidazopyridine–propenone conjugates (8a–8u) were synthesized and evaluated for their antitumor activity against four human cancer cell lines, namely, prostate (DU-145), lung (A549), cervical (Hela) and breast (MCF-7) cancer cell lines. These conjugates showed good to moderate activity against the tested cell lines. Among them, two conjugates (8m and 8q) showed significant antiproliferative activity against the human lung cancer cell line (A549) with IC50 values of 0.86 μM and 0.93 μM, respectively. Flow cytometry analysis revealed that these compounds arrested the cell cycle at the G2/M phase in the human lung cancer cell line (A549), inhibiting tubulin polymerization leading to apoptosis. Further, Hoechst staining, decrease in mitochondrial membrane potential and Annexin V-FITC assay suggested that the cell death was due to apoptosis induction. Overall, the present investigation demonstrated that the synthesized imidazopyridine–propenone conjugates are promising tubulin inhibitors and apoptotic inducers.


 Please wait while we load your content...
Please wait while we load your content...