Synthesis and in vitro study of novel borneol derivatives as potent inhibitors of the influenza A virus†‡
Abstract
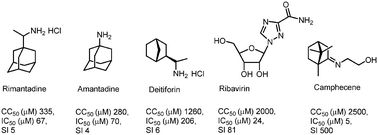
Herein, we present the design and synthesis of a series of novel heterocyclic derivatives of (−)-borneol and (−)-isoborneol as potent inhibitors of the influenza A virus. All compounds were tested for their toxicity against MDCK cells and for virus-inhibiting activity against the influenza virus A/Puerto Rico/8/34 (H1N1). Compounds 7, 16 and 26 containing a morpholine fragment exhibited the highest efficiency as agents inhibiting the replication of the influenza virus A(H1N1) with selectivity indices of 82, 45 and 65, correspondingly. Derivatives 9 (SI = 23) and 18 (SI = 25) containing a 1-methylpiperazine motif showed moderate antiviral activity. Structure–activity analysis of this new series of borneol derivatives revealed that a 1,7,7-trimethylbicyclo[2.2.1]heptan scaffold is required for the antiviral activity.


 Please wait while we load your content...
Please wait while we load your content...