Development of new scaffolds as reversible tissue transglutaminase inhibitors, with improved potency or resistance to glutathione addition†‡
Abstract
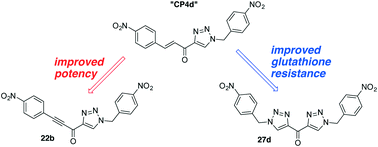
Previous studies within our group have yielded a class of cinnamoyl-based competitive reversible inhibitors for tissue transglutaminase (TG2), with Ki values as low as 1.0 μM (compound CP4d). However, due to the electrophilic nature of their alkene moiety, this class of inhibitors is susceptible to nucleophilic attack by glutathione, a key element in cellular metabolism and toxicity response. To address this issue, we made several modifications to the inhibitor scaffold, ultimately showing that a bis(triazole) scaffold increased resistance to nucleophilic attack, with compound 27d being the most potent (Ki = 10 μM). In the process of reducing reactivity, we also prepared a new class of inhibitors, replacing the alkene of CP4d with an alkyne, leading to a significant increase in potency for compound 22b (Ki = 420 nM).


 Please wait while we load your content...
Please wait while we load your content...