Synthesis and biological activity evaluation of novel peroxo-bridged derivatives as potential anti-hepatitis B virus agents†
Abstract
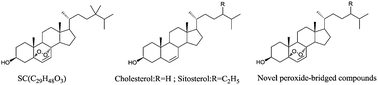
Previous studies have demonstrated that natural steroid compounds containing a peroxide bridge exhibited potential anti-hepatitis B virus activity. To continue our research, a simple and regioselective methodology, using Eosin Y as a clean photosensitized oxidation catalyst, was developed for the synthesis of a peroxide bridge in steroids. The method that using Eosin Y as the catalyst was exposed to visible light and furbished in high yields, did not involve tedious work-up or purification, and avoided using environmentally hazardous solvents. It can be regarded as a green protocol. Moreover, a series of cholesterol and β-sitosterol derivatives containing a peroxide bridge were synthesized using this method and screened for their anti-HBV activity. Among the compounds synthesized in this research, 5α,8α-cyclicobioxygen-6-vinyl-3-oxo-cholesterone (1f, 3.13 μg ml−1) had the most potent activity with inhibition rates of 77.45% ± 6.01% and 58.73% ± 8.64% on the secretion of HBsAg and HBeAg antigens, respectively, after 8 days. Further acute toxicity test showed that the LD50 value of compound 1f was 362.46 mg kg−1 after an intraperitoneal injection in mice. Moreover, structure–activity relationships of cholesterol and β-sitosterol derivatives were briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...