Abstract
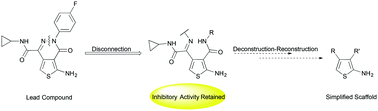
Aminothienopyridazines (ATPZs) have demonstrated efficacy, in vitro, as tau protein aggregation inhibitors. Modifications were made to the ATPZ scaffold to determine the importance of certain structural features for activity. More specifically, ring-opened analogues detached at the nitrogen–nitrogen bond of the pyridazine, were synthesized and their inhibitory activity evaluated. Preliminary data suggests that the ring-opened structures retain inhibitory activity, independent of tau oxidation. The structures detailed represent the beginnings of a deconstruction–reconstruction–elaboration study, with the aim of identifying simpler scaffolds, which retain activity and can be optimized in terms of physiochemical properties.


 Please wait while we load your content...
Please wait while we load your content...