Abstract
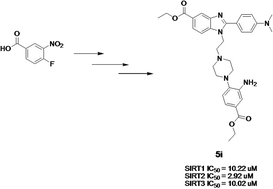
Two series of compounds were synthesized based on the benzimidazole scaffold. The compounds were subsequently screened for their SIRT1, SIRT2 and SIRT3 activities. Three of the compounds showed good inhibitory activity against SIRT2 in this study with the most potent compound (5i) having an IC50 value of 2.9 μM. Molecular docking analysis demonstrated that 5i was able to inhibit SIRT2 by displacing the co-factor NAD+ in the active site. This was further confirmed experimentally by ligand-NAD+ competitive assay.

 Please wait while we load your content...
Please wait while we load your content...