Improved antiviral activity of a polyamide against high-risk human papillomavirus via N-terminal guanidinium substitution†‡
Abstract
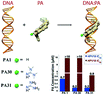
We report the synthesis of two novel pyrrole–imidazole polyamides with N-terminal guanidinium or tetramethylguanidinium groups and evaluate their antiviral activity against three cancer-causing human papillomavirus strains. Introduction of guanidinium improves antiviral activity when compared to an unsubstituted analog, especially in IC90 values. These substitutions change DNA-binding preferences, while binding affinity remains unchanged.


 Please wait while we load your content...
Please wait while we load your content...