Synthesis and preliminary evaluation of novel alkyl diamine linked bivalent β-carbolines as angiogenesis inhibitors†‡
Abstract
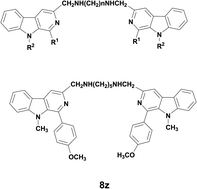
A series of novel bivalent β-carbolines linked with an alkyl diamine spacer at the C-3 position were synthesized and evaluated as potent angiogenesis inhibitors. The results demonstrated that most bivalent β-carbolines displayed significant anti-proliferative effects against EA.HY926 human umbilical vein cell lines. Compound 8z was found to be the most potent anti-proliferative agent with an IC50 value of 1.10 μM against EA.HY926 cell lines. Preliminary investigations on the mechanisms of action revealed that compound 8z could dramatically inhibit EA.HY926 cell migration and tube formation in a dose-dependent manner. Moreover, compound 8z exhibited significant angiogenesis inhibitory effects in CAM assay, and the anti-angiogenic potency was comparable with that of the reference drug Endostar. These molecules might serve as candidates for further development into vascular-targeting antitumor drugs.

 Please wait while we load your content...
Please wait while we load your content...