The discovery of kinase inhibitors by a combination of diversity-oriented synthesis and selective screening†‡
Abstract
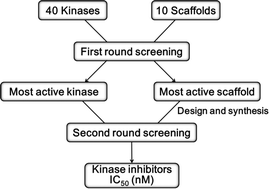
An efficient strategy for the discovery of kinase inhibitors by a combination of diversity-oriented synthesis and selective screening is described. Initially, a set of 10 compounds representing a 10-scaffold library of diversity-oriented pyrimidine-containing compounds were tested against 40 enzymes selected from the kinase family, which resulted in the discovery of a selective p38α inhibitor. In the next step, the subset from the scaffold with the active was screened against the pairing enzyme (p38α). Eventually, the most potent compounds were measured for IC50 (42.7 nM) and tested in MM1S and U266 cells by observing the complete suppression of the phosphorylation of p38α at submicromolar concentrations. Therefore, a series of novel tricyclic pyrimido[4,5-b][1,4]benzothiazepines with potent inhibitory activity against p38α kinase in vitro and in cellular assays were discovered utilizing this methodology. This strategy may be applicable for the drug discovery of a superfamily of targets using a large library of compounds.

 Please wait while we load your content...
Please wait while we load your content...