Abstract
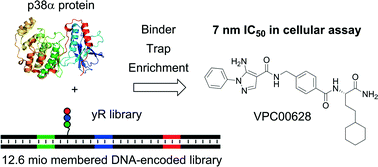
A highly specific and potent (7 nM cellular IC50) inhibitor of p38α kinase was identified directly from a 12.6 million membered DNA-encoded small molecule library. This was achieved using the high fidelity yoctoReactor technology (yR) for preparing the DNA-encoded library, and a homogeneous screening technique – the binder trap enrichment technology (BTE). Although structurally atypical to other kinase blockers, this inhibitor was found by X-ray crystallography to interact with the ATP binding site and provide strong distortion of the P-loop. Remarkably, it assumed an alternative binding mode as it lacks key features of known kinase inhibitors such as typical hinge binding motifs. Interestingly, the inhibitor bound assuming a canonical type-II (‘DFG-out’) binding mode by forming hinge hydrogen bonds with the backbone, showed excellent shape complementarity, and formed a number of specific polar interactions. Moreover, the crystal structure showed, that although buried in the p38α active site, the original DNA attachment point of the compound was accessible through a channel created by the distorted P-loop conformation. This study demonstrates the usability of DNA-encoded library technologies for identifying novel chemical matter with alternative binding modes to provide a good starting point for drug development.

 Please wait while we load your content...
Please wait while we load your content...