Novel heterocyclic ring-fused oleanolic acid derivatives as osteoclast inhibitors for osteoporosis†‡
Abstract
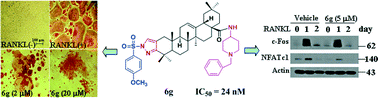
Osteoporosis is a major public health problem in our aging society. In the present study, a series of novel oleanolic acid (OA) derivatives were synthesized via modifications in the A-ring and C-28 position of OA, and their anti-bone resorption activities were evaluated. Screening results revealed that most of the derivatives inhibited RANKL-induced osteoclast formation from RAW264.7 cells. Among the derivatives, 6g exhibited a potent inhibitory activity with an IC50 of 24 nM. Furthermore, 6g prevented ovariectomy-induced bone loss in rats. The preliminary mechanism investigation demonstrated that 6g suppressed the protein and mRNA expressions of c-Fos and NFATc1, and downregulated the mRNA of other osteoclastogenic markers. The results suggested that the OA derivatives might serve as potential leads for the development of novel agents for the treatment of osteoporosis.

 Please wait while we load your content...
Please wait while we load your content...